Higher-Valent Nickel Oxides with Improved Oxygen Evolution Activity and Stability in Alkaline Media Prepared by High-Temperature Treatment of Ni(OH) 2 | ACS Catalysis

Synthesis of Hexagonal Nickel Hydroxide Nanosheets by Exfoliation of Layered Nickel Hydroxide Intercalated with Dodecyl Sulfate Ions | Journal of the American Chemical Society
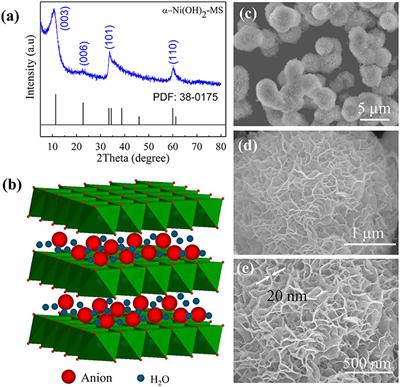
Frontiers | Facile Synthesis of Monodispersed α-Ni(OH)2 Microspheres Assembled by Ultrathin Nanosheets and Its Performance for Oxygen Evolution Reduction

QUESTIONFind out the solubility of Ni(OH) 2 in 0.1 M NaOH. Given that the ionic product of Ni(OH)2 is 2×10 - Brainly.in

Figure 5 from Thermodynamic model of Ni(II) solubility, hydrolysis and complex formation with ISA | Semantic Scholar

One material, multiple functions: graphene/Ni(OH)2 thin films applied in batteries, electrochromism and sensors | Scientific Reports

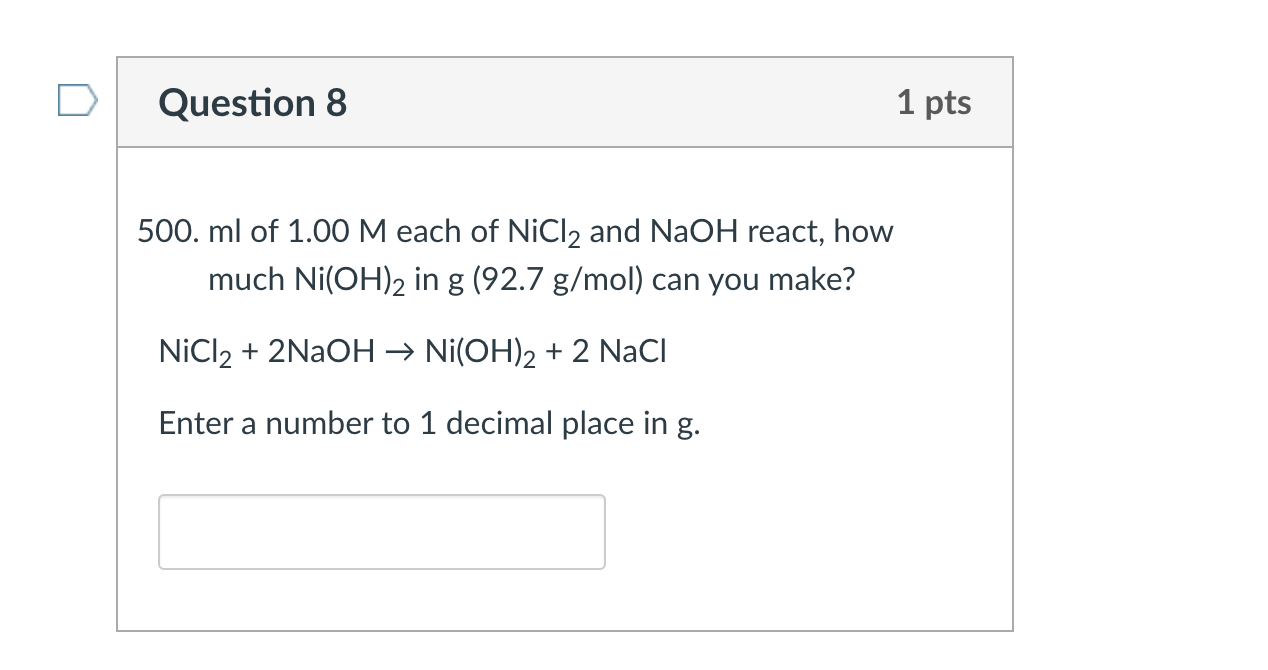
Calculate the molar solubility of Ni(OH)2 in 0.10 M NaOH solution. The ionic product of Ni(OH)2 2.0 × 10^-15

Porous Fe-Doped β-Ni(OH)2 Nanopyramid Array Electrodes for Water Splitting | ACS Applied Materials & Interfaces

Ionic product of Ni(OH)2is 2.0 x 10-15Molar solubility of Ni(OH)2in 0.10 M NaOH will bea)1.0 x 10-13Mb)2.0 x 10-13Mc)4.0 x 10-13Md)8.0 x 10-13MCorrect answer is option 'B'. Can you explain this answer? -

STA curves: (A) DSC and (B) TGA for reflux-Ni(OH)2 and RT NaOH-Ni(OH)2.... | Download Scientific Diagram

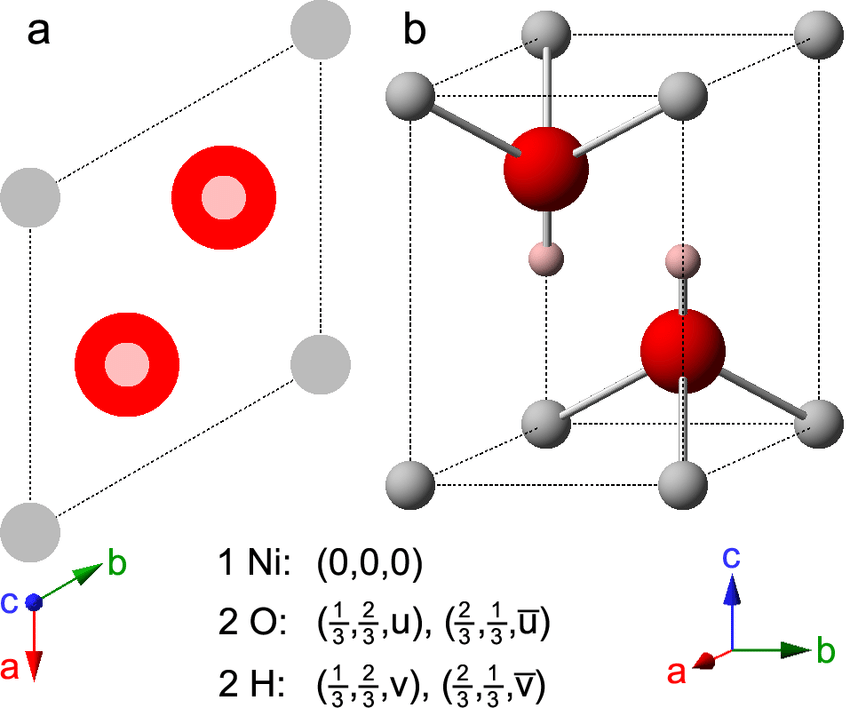
Nickel hydroxides and related materials: a review of their structures, synthesis and properties | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences
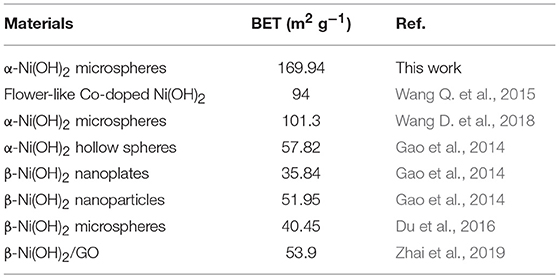
Frontiers | Facile Synthesis of Monodispersed α-Ni(OH)2 Microspheres Assembled by Ultrathin Nanosheets and Its Performance for Oxygen Evolution Reduction

Nickel hydroxide precipitate formed by adding sodium hydroxide (NaOH) to a solution containing nickel ions. Nickel hydroxide (Ni(OH)2) is precipitated Stock Photo - Alamy





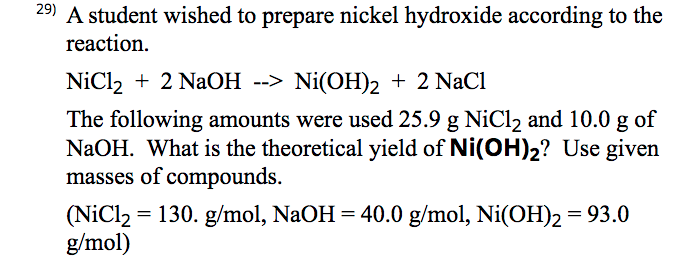
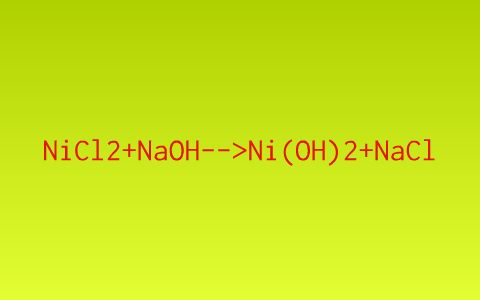

![Malayalam] Calculate the solubility of Ni(OH)2 in 0.1M NaOH solution. Malayalam] Calculate the solubility of Ni(OH)2 in 0.1M NaOH solution.](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/6531163.webp)