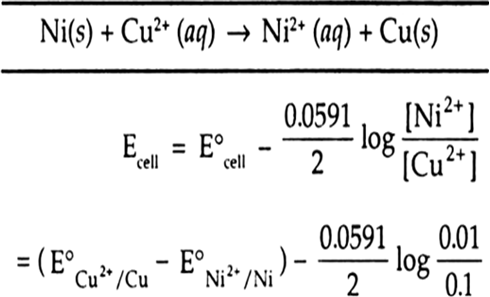Three-Dimensional Interconnected Binder-Free Mn–Ni–S Nanosheets for High Performance Asymmetric Supercapacitor Devices with Exceptional Cyclic Stability | ACS Applied Energy Materials

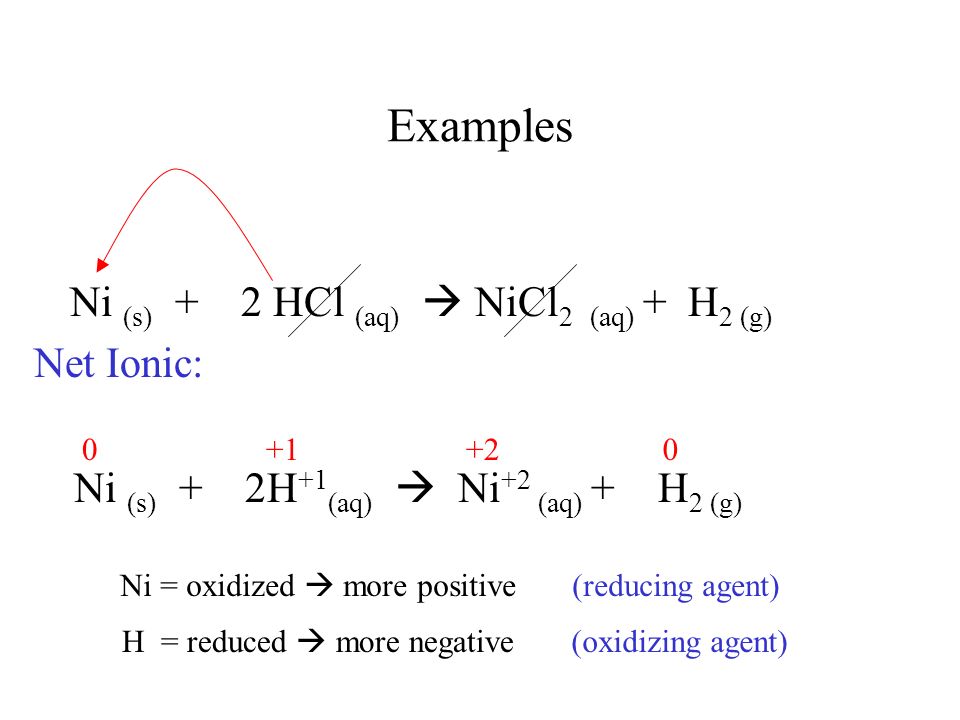
Question Video: Determining Whether a Substance Is an Oxidizing or Reducing Agent from a Chemical Equation | Nagwa

Interface Engineering of NixSy@MnOxHy Nanorods to Efficiently Enhance Overall-Water-Splitting Activity and Stability | Nano-Micro Letters

One-Step Synthesis of Nickel Sulfides and Their Electrocatalytic Activities for Hydrogen Evolution Reaction: A Case Study of Crystalline h-NiS and o-Ni9S8 Nanoparticles | ACS Applied Energy Materials

Surface Activation and Ni‐S Stabilization in NiO/NiS2 for Efficient Oxygen Evolution Reaction - Zhang - 2022 - Angewandte Chemie International Edition - Wiley Online Library

4 15. E.M.F. of Ni(s)[Ni2+ (aq) || Cu2+ (aq)|Cu(s) cell can be increased by (1) Adding NH, in the right half-cell (2) Increasing the conc. of Ni2+ ions (3) Adding dimethyl

Design of a carbon-resistant Ni@S-2 reforming catalyst: Controllable Ni nanoparticles sandwiched in a peasecod-like structure - ScienceDirect

Calculate the emf of the cell in which the following reaction takes place: Ni(s)+2Ag^+(0.002M)→ Ni^2+(0.160 M)+2Ag(s) Given that E^∘_cell=1.05 V nbsp;

Calculate the `EMF` of the cell in whiCHM the following reaction takes place `:` `Ni(s)+2Ag^(o+)... - YouTube

What does NI(S) mean? - Definition of NI(S) - NI(S) stands for Naval Instructions to Salaried Consular Officers. By AcronymsAndSlang.com

Surface Restructuring of Nickel Sulfide Generates Optimally Coordinated Active Sites for Oxygen Reduction Catalysis - ScienceDirect
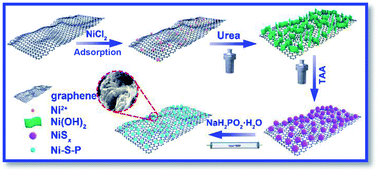
Anionic P-substitution toward ternary Ni–S–P nanoparticles immobilized graphene with ultrahigh rate and long cycle life for hybrid supercapacitors - Journal of Materials Chemistry A (RSC Publishing)

The FTIR spectra of NH2-NI/S and NH2-NI/S after adsorption of Pb(II)... | Download Scientific Diagram

Heterogeneous histories of Ni‐bearing pyrrhotite and pentlandite grains in the CI chondrites Orgueil and Alais - Berger - 2016 - Meteoritics & Planetary Science - Wiley Online Library
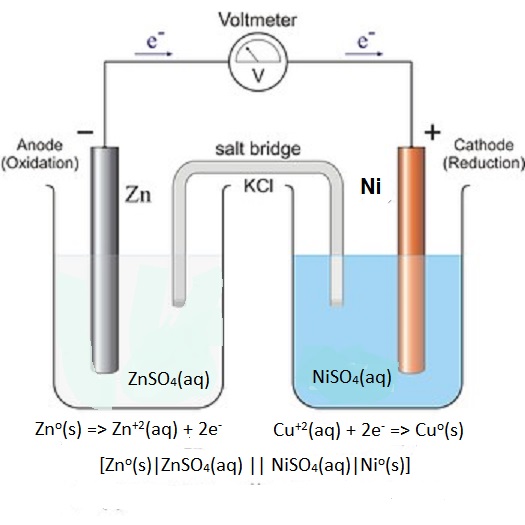
Zn(s) +Ni^(2+)(aq) -> Zn^(2+)(aq) + Ni(s) Which part of the cell conducts electrons in this reaction and describes the direction of electron flow as the cell operates? | Socratic





![PDF] Raman Spectroscopy of Nickel Sulfide Ni | Semantic Scholar PDF] Raman Spectroscopy of Nickel Sulfide Ni | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/345ef7d84582812e979cbd9a8f6545615d4ef1a4/2-Figure4-1.png)